Introduction: Suppression of tumorigenicity 2 (ST2) is a prognostic plasma marker of non-relapse mortality (NRM) after allogeneic hematopoietic cell transplantation (allo-HCT) when measured at day +14 [Vander Lugt MT et al., N Engl J Med, 2013] and at day +7 in combination with regenerating islet-derived 3α (REG3α) [Hartwell MJ et al., JCI Insight, 2017]. We hypothesized that also pre-transplantation ST2 levels would be associated with NRM in the first 6 months after allo-HCT.
Methods: We studied 112 adult patients who underwent allo-HCT with myeloablative conditioning at Rigshospitalet between July 2015 and August 2018 (Table 1). ST2 levels were measured by enzyme-linked immunosorbent assays using stored EDTA plasma samples obtained at the patients' scheduled pre-transplantation visit around day -23 (±11 days) and post-transplantation at days +7 and +14 (±3 days, n = 76 and 66). Univariable linear models and Spearman's ρ were used to evaluate associations and correlations between pre-transplantation ST2 levels and patient characteristics and other prognostic markers, respectively. Cause-specific Cox regression models were used to estimate hazard ratios (HR) with 95% confidence intervals (CI) for NRM in the first 6 months after allo-HCT (relapse as competing risk) and grade II-IV acute graft-versus-host disease (GvHD) in the first 100 days after allo-HCT (NRM and relapse as competing risks) according to pre-transplantation ST2 levels. Gray's test was used to test differences in the cumulative incidence of NRM in the first 6 months after allo-HCT according to quartiles of pre-transplantation ST2 levels.
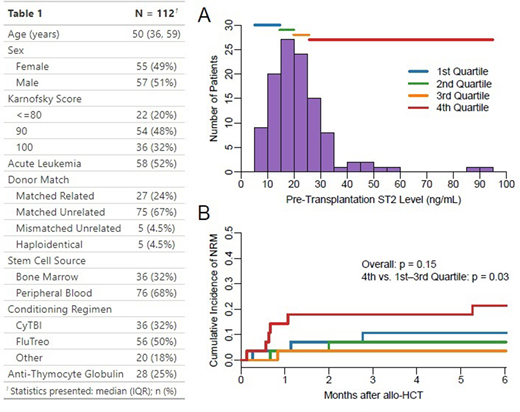
Results: The median pre-transplantation plasma ST2 level was 19.9 ng/mL (inter-quartile range: 14.6-25.7 ng/mL, Figure Panel A); levels were higher in males (β = 8.7 ng/mL, p < 0.01), but did not differ by age (p = 0.81) or by being transplanted for acute leukemia (p = 0.89). ST2 was correlated with C-reactive protein (ρ = 0.24, p = 0.01), the endothelial activation and stress index (EASIX, calculated as creatinine x lactate dehydrogenase/thrombocytes, ρ = 0.27, p < 0.01) and ferritin (ρ = 0.28, p < 0.01). Longitudinally, pre-transplantation ST2 levels were strongly correlated with ST2 levels on day +7 (ρ = 0.57, p < 0.01) and day +14 (ρ = 0.48, p < 0.01). The cumulative incidence of NRM at 6 months was 11% (n = 12); causes of death were organ failure (75%), acute graft-versus-host disease (GvHD, 17%) and infection (8%). Higher pre-transplantation ST2 levels were associated with increased hazard of NRM in the first 6 months after allo-HCT (HR 1.73 per 10 ng/mL increase, 95% CI: 1.28-2.33, p < 0.01, area under the receiver operating characteristics curve = 0.61). Despite a significantly higher NRM in patients with pre-transplantation ST2 levels in the highest quartile (cumulative incidence at 6 months: 21% vs. 7% in patients with levels in the three lower quartiles, p = 0.03), there was no overall significant difference in NRM according to quartiles of pre-transplantation ST2 levels (p = 0.15, Figure Panel B). No significant association was found between pre-transplantation ST2 levels and grade II-IV acute GvHD (HR 0.88, 95% CI: 0.61-1.26, p = 0.48).
Conclusion: Pre-transplantation ST2 levels were associated with increased NRM in the first 6 months after myeloablative allo-HCT, mainly driven by higher NRM in patients with pre-transplantation ST2 levels in the highest quartile. Larger studies are warranted to validate pre-transplantation ST2 levels as a prognostic marker of NRM after allo-HCT, which potentially could be used to support the choice of conditioning intensity.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.